FOR UK HEALTHCARE PROFESSIONALS ONLY
Please click here for Prescribing Information and further information about Staladex. Adverse event reporting can be found at the bottom of the page.
Dosage and Administration
Designed to save time
Staladex is a ready-to-use leuprorelin implant which is injected subcutaneously under the abdominal skin once every three months.
Watch the short video below to see how to administer Staladex to your patients in 7 easy steps.
Nurse Training
Aspire Pharma is committed to the training of healthcare professionals in the administration of Staladex.
Training can be provided online via Microsoft teams, or in person at a convenient location for either individuals or groups, by a third party training provider.
Engineered for precision
The Staladex needle is designed for precision and patient comfort.
Legal Class: POM
Active Substances: Each implant contains 11.25 mg leuprorelin acetate (equivalent as 10.72 mg leuprorelin)
Price: £206.00 for a 3-monthly implant
Staladex can be implanted in 7 easy steps
that have been designed to save time vs. reconstitution:
One
Remove the applicator from the sterile pouch.
The pre-filled syringe must be used immediately after opening the pouch.
Check whether the implant is in the intended position in the applicator.
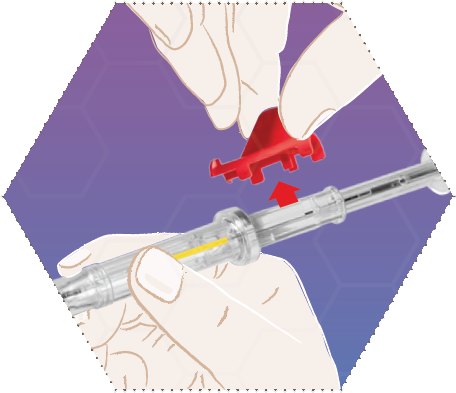
Remove the red safety ring.
Two
Grip the applicator on the syringe barrel and remove the protective cover without touching the needle.
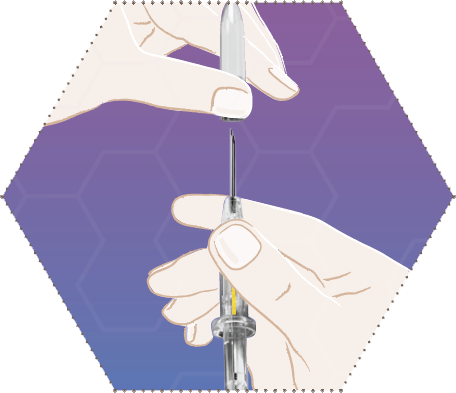
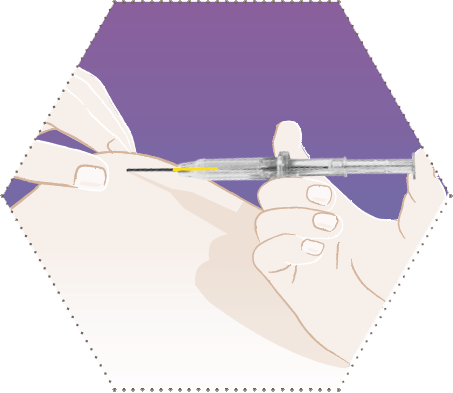
Three
Press the skin of the patient together while holding the syringe barrel and insert the needle obliquely (almost parallel to the skin) with the opening of the needle facing up.
Insert the needle into the subcutaneous tissue (not in muscle or into the abdominal cavity) of the anterior abdominal wall under the umbilical line, until the syringe barrel touches the patient’s skin.
The syringe barrel must remain in contact with the skin during the entire application process.
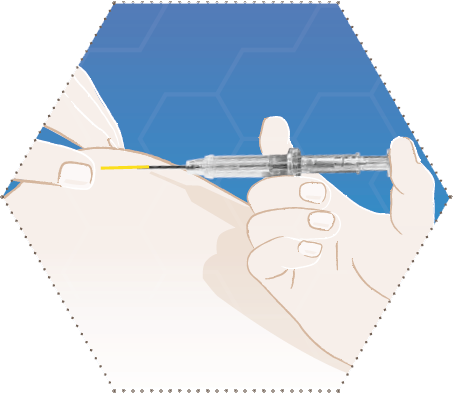
Four
Press the syringe plunger down.
The implant is transported to the needle tip. Do not pull the syringe back.
During the application, the syringe barrel has to touch the patient’s skin.
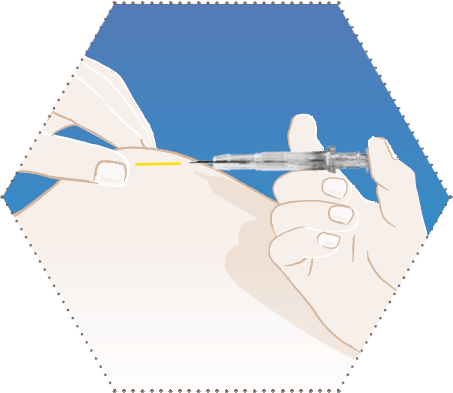
Five
When the plunger is stopped, the needle retraction is unlocked automatically.
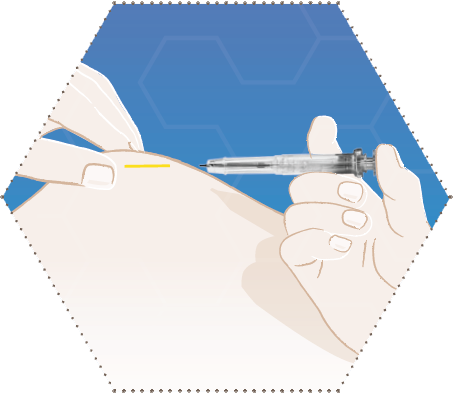
Six
The needle is retracted from the tissue into the syringe barrel.
The syringe barrel must remain in contact with the patient’s skin.
Normally, the plunger movement forwards and the needle retraction are carried out in one smooth motion.
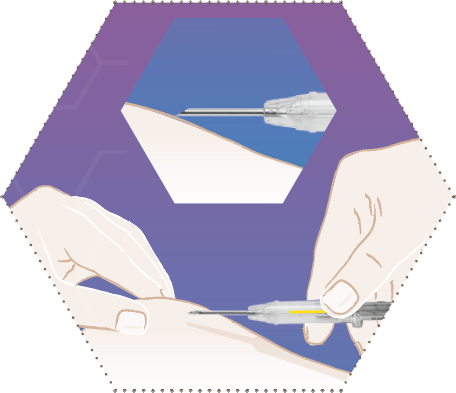
Seven
The application process is completed. The needle has been fully retracted into the barrel of the syringe.
The supernatant mandrel protects against injury at the needle tip.
STA1010606D4_FEB2026