FOR UK HEALTHCARE PROFESSIONALS ONLY
Please click here for Prescribing Information and further information about Staladex. Adverse event reporting can be found at the bottom of the page.
Efficacy and Safety
Inconsistency can impact efficacy
Handling errors in the preparation of leuprorelin depots may result in some patients receiving insufficient amounts of medicine. Errors can include incorrect use of the needle or syringe, causing the medicine to leak from the syringe, and failure to inject leuprorelin properly.1
Errors with the preparation and administration of leuprorelin containing depot injections can lead to under-dosing, potentially resulting in a lack of efficacy.1
The risk of error is increased when there are multiple steps in the reconstitution and administration process.1
Staladex offers a consistent clinical profile
Efficacy:
An open label, one-arm, multiple dose Phase III clinical study* in patients with prostate cancer investigated the clinical efficacy and safety of Staladex.2
With Staladex, 94% (47/50) of patients achieved consistent testosterone suppression below castrate level from visit Day 28 until the end of the study at Day 168 with the corresponding exact 95% confidence interval [83.45%; 98.75%].3
Safety:
Staladex was found to be generally well tolerated.3
Release Profile
Following injection of the implant, leuprorelin acetate is released continuously over a period of 3 months.
Repeated dosing produces a sustained reduction in the testosterone concentration to castration levels, without the testosterone concentration showing the transient rise seen after the first injection.4
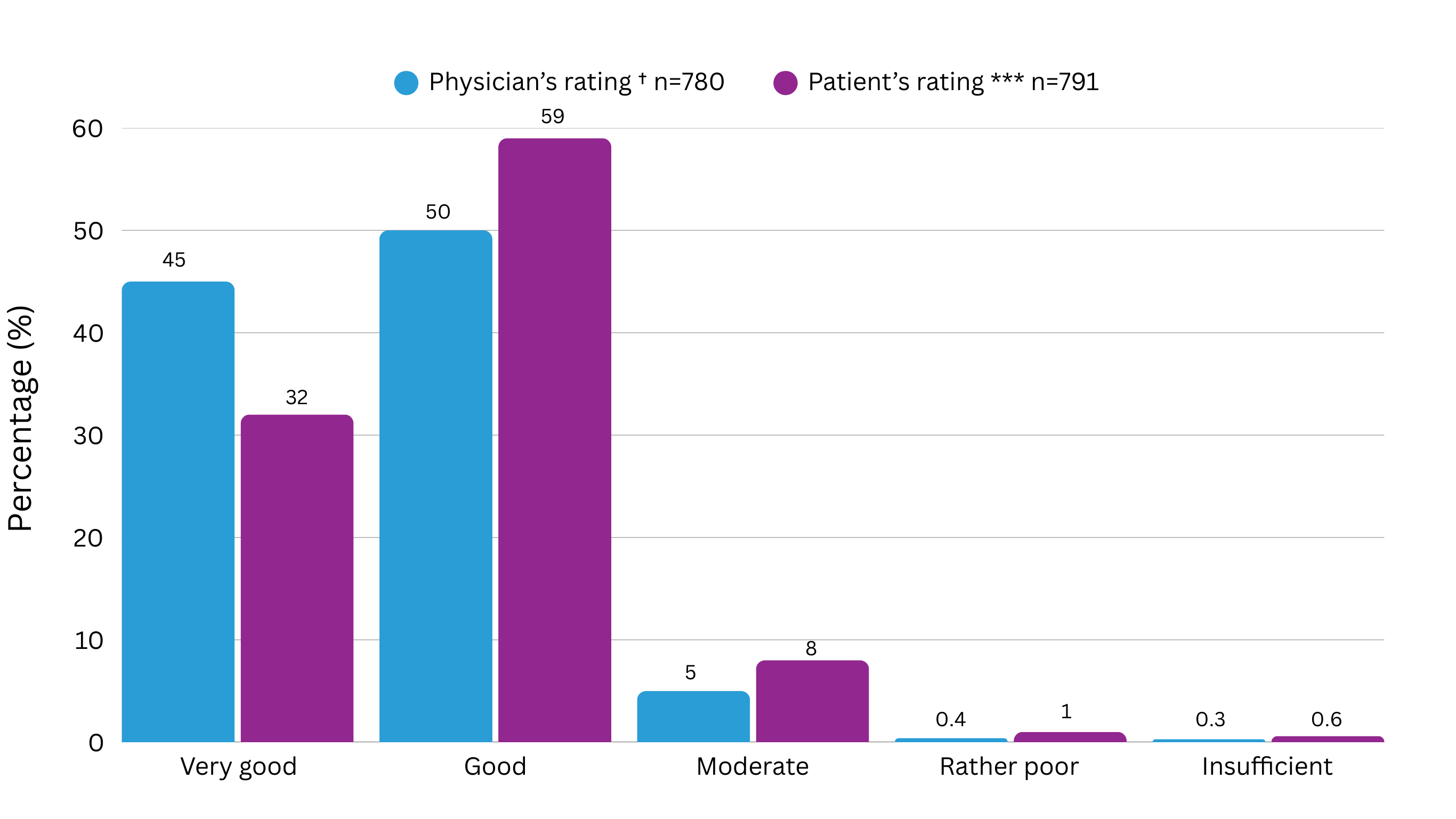
HCP satisfaction with a leuprorelin implant:
A study assessed the efficacy and tolerability of two leuprorelin implants** and found that5:
- Physicians were either ‘very satisfied’ or ‘satisfied’ with the treatment outcome in 76% of patients (n=572).
- Tolerability of the leuprorelin implants was generally assessed as very good/good by physicians for 95% of their patients (n=739) and by the patients themselves for 91% (n=716).
** 1 and 3 month leuprorelin implants containing 3.6 mg and 5 mg of leuprorelin acetate respectively. The study did not include Staladex®
† Based on patients with assessment of tolerability (n=780)
*** Based on patients with assessment of tolerability (n=791)
Footnotes
* Primary study objective was to demonstrate that Staladex® lead to consistent suppression of testosterone levels below castrate level (0.5 ng/mL). Testosterone plasma levels were measured by liquid chromatography-mass spectrometry/mass spectrometry at Day 28, 42, 56, 70, 84, 87, 98, 112, 126, 140, 154 and 168. Adverse events and vital signs were monitored throughout treatment phase.
References
- European Medicines Agency 2020. New measures to avoid handling errors with leuprorelin depot medicines. Available at: https://www.ema.europa.eu/en/documents/press-release/leuprorelin-depot-medicines-prac-recommends-new-measures-avoid-handling-errors_en.pdf (Accessed March 2025)
- Phase III trial of Staladex implant, EU Clinical Trials Register; Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-006014-14/results (Accessed March 2025)
- Leuprorelin Amdeepcha, Public Assessment Report-2019; Available from: https://mri.cts-mrp.eu/portal/details?productnumber=DE%2FH%2F5485%2F001#:~:text=PAR%20%7C%2001_DE5485_1_DC%20Leuprorelin%20Amdeepcha_draft%20PAR (Accessed March 2025)
- Staladex SmPC
- Gravel, P., Samland, D., Loeffler, M., Maier, S., Panozzo, M. and Muenzberg, M., 2013. Two innovative pharmaceutical forms of leuprorelin: results from 818 patients with advanced prostate cancer. Advances in therapy, 30(3), pp.271-285
STA1010606C1_MAR2025