FOR UK HEALTHCARE PROFESSIONALS ONLY
Please click here for Prescribing Information and further information about Staladex. Adverse event reporting can be found at the bottom of the page.
Consistency doesn’t have to be boring!
Staladex® – the consistent choice.
Staladex 11.25mg leuprorelin acetate (equivalent as 10.72mg leuprorelin) is a long-acting implant formulation of leuprorelin that is indicated in men for the treatment of 1:
- Metastatic prostate cancer
- Locally advanced prostate cancer, as an alternative to surgical castration
- As an adjuvant treatment to radiotherapy in patients with high-risk localised or locally advanced prostate cancer.
- As an adjuvant to radical prostatectomy in patients with locally advanced prostate cancer at high risk of disease progression
- As neo-adjuvant treatment prior to radiotherapy in patients with high-risk localised or locally advanced prostate cancer
Keep things consistent with Staladex:
With its implant-based delivery system, Staladex is a ready-to-use 3 monthly leuprorelin implant which helps to ensure consistent dosing by removing the need for reconstitution prior to injection.1
Staladex can be implanted in 7 easy steps that have been designed to save time vs. reconstitution.
Inconsistency can impact efficacy
- Errors with the preparation and administration of leuprorelin containing depot injections can lead to under-dosing, potentially resulting in a lack of efficacy.2
- The risk of error is increased when there are multiple steps in the reconstitution and administration process.2
Designed to save…
In a study, it was estimated that the leuprorelin implant saved a total of approximately 30 hours of labour time per 1000 patients per year when compared with three common LHRH agonist formulations due to less preparation time.4
In a cost assessment study conducted in Europe, a leuprorelin solid implant* was associated with potential administration cost savings compared with the most commonly used LHRH agonist preparations.4
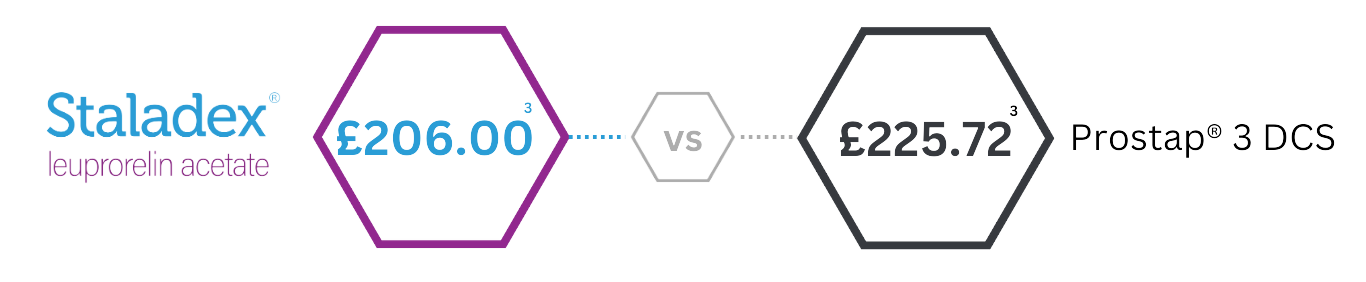
Staladex® is now the least expensive 3-monthly LHRH agonist available in the UK.3
Footnotes
* Leuprorelin–Sandoz® was used for purposes of investigation
References
- Staladex SmPC (Accessed March 2025)
- European Medicines Agency 2020. New measures to avoid handling errors with leuprorelin depot medicines. Available at: https://www.ema.europa.eu/en/documents/press-release/leuprorelin-depot-medicines-prac-recommends-new-measures-avoid-handling-errors_en.pdf (Accessed March 2025)
- UK Drug Tariff (Accessed March 2025)
- Merseburger, A.S., Björk, T., Whitehouse, J. and Meani, D., 2015. Treatment costs for advanced prostate cancer using luteinizing hormone-releasing hormone agonists: a solid biodegradable leuprorelin implant versus other formulations. Journal of comparative effectiveness research, 4(5), pp.447-453.
STA1010606B1_MAR2025